Online Services
Research Ethics Committee
NCMH Research Ethics Committee
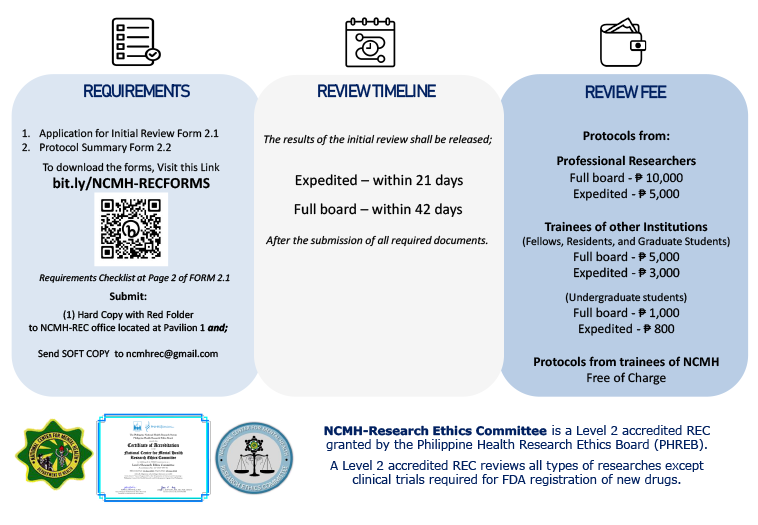
NCMH-Research Ethics Committee is a Level 2 accredited REC granted by the Philippine Health Research Ethics Board (PHREB).
A Level 2 accredited REC reviews all types of researches except clinical trials required for FDA registration of new drugs.
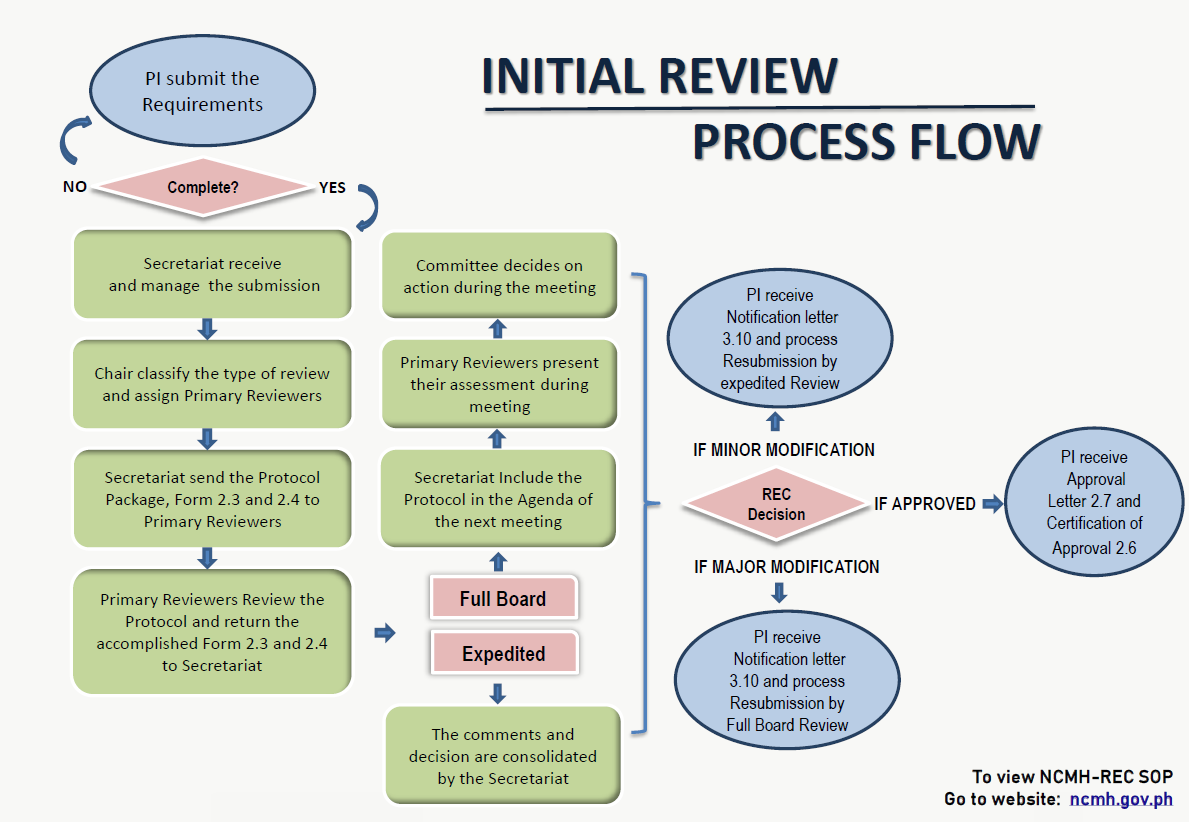
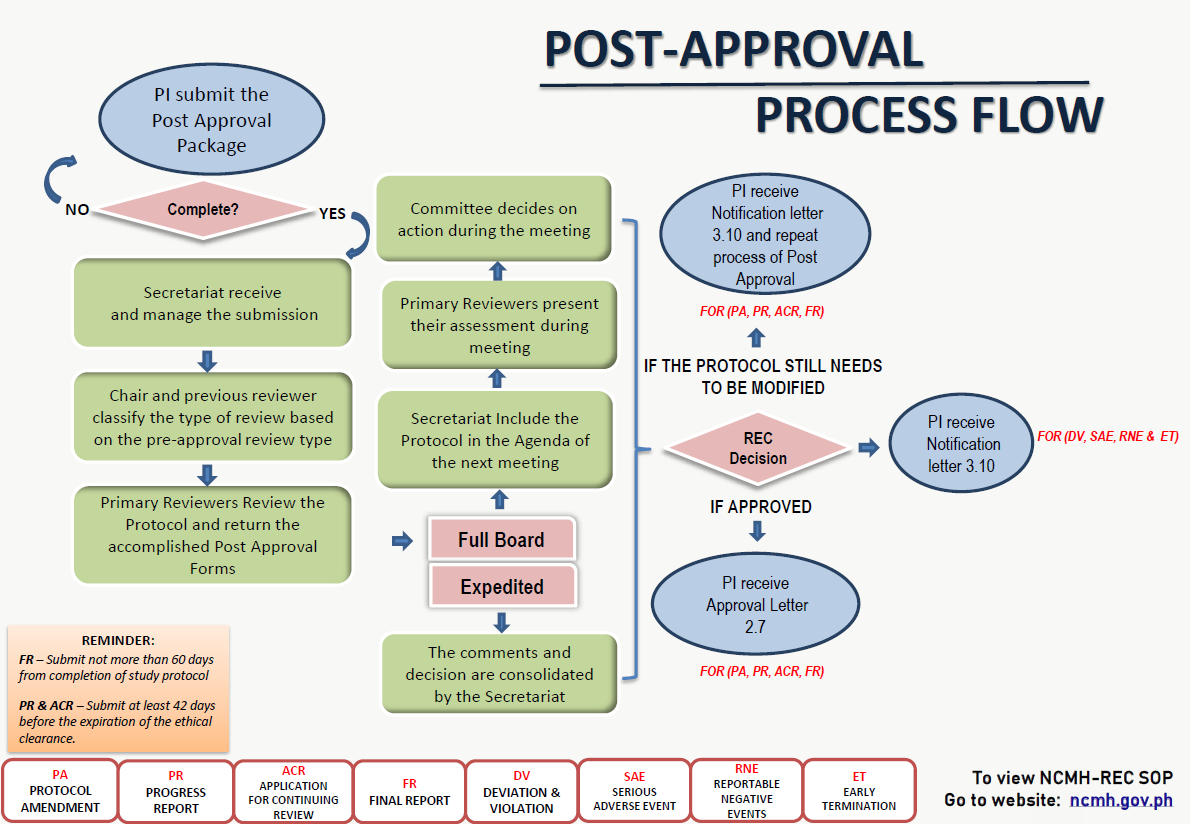
NCMH-Research Ethics Committee (REC) is a body that makes independent decisions regarding the review, approval, and implementation of research protocols/proposals, in order to ensure the protection of the rights, safety, and well-being of human participants and promotes integrity of research data. It shall be constituted by a duly recognized authority and shall adhere to national and international research ethics guidelines.
Mission & Vision
VISION
“The NCMH-REC aims to be the premier research ethics committee that advances the mental health and well-being, the rights and interest of every participant and soon to be the reference center of research in terms of mental health.”
MISSION
“To protect the Rights, Safety, Welfare, and Confidentiality of Human Research participants through efficient and effective review process by upholding the highest Ethical Standards in Research.”
NCMH-REC Forms
Application for Initial Review Form 2.1
Protocol Summary Sheet Form 2.2
Protocol Evaluation Form 2.3
Informed Consent Evaluation Form 2.4
Protocol Resubmission Form 2.5
Protocol Amendment Form 3.1
Progress Report Form 3.2
Application for Continuing Review Form 2.9
Early termination Form 3.8
Protocol Violation/Deviation Report Form 3.5
Query/Complaint Record Form 3.6
Onsite Serious Adverse Event Report Form 3.4